This reacts reversibly with iodine to give a deep blue starch-iodine complex which is much easier to see. Preparation of Lyophilic and Lyophobic Sols.

What Happens When Hydrochloric Acid Sodium Thiosulphate React

Reaction Of Sodium Thiosulphate And Hydrochloric Acid Ppt Powerpoint
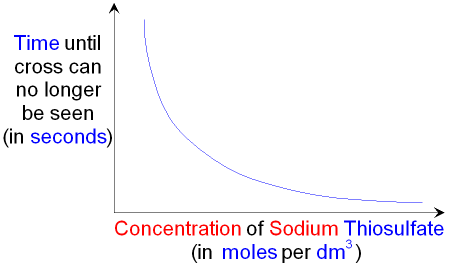
Gcse Chemistry What Is The Effect Of Increasing The Concentration On The Rate Of A Chemical Reaction Collision Theory Gcse Science
Sodium thiosulphate and hydrochloric acid.
Sodium thiosulphate and hydrochloric acid. Take up a quiz on Effect of concentration on the rate of reaction between sodium thiosulphate and hydrochloric acid. The temperature must be measured after adding the acid because the cold acid cools the solution slightlyThis time the temperature is changed between experiments keeping everything else constant. It is a common substance found in rocks in all parts of the world and is the main component of shells of marine organisms.
Eriochrome Blue Black r. Sodium thiosulphate Thiosulfate de sodium Sodium hyposulphite. Kinetics Study on the Reaction between Sodium Thiosulphate and Hydrochloric Acid.
Sodium Thiosulphate Hydrochloric acid Sulphur Sodium Chloride Sulphur Dioxide Water. Hydrogen peroxide Peroxyde dhydrogène Hydrogen dioxide Hydroperoxide. Acidic emission control sludges from secondary lead smelting.
Make up the volume 1000 ml with water. Put your understanding of this concept to. Prepare the following solutions Hydrochloric acid 01 mol dm-3 Sodium thiosulphate 1 mol dm-3 You will need to think about how much of each solution to prepare.
When it is almost all gone you add some starch solution. Calcium carbonate is a chemical compound with the formula CaCO3. Supply of acetone gr grade in 25 litre bottle iso propyl alcohol argf grade in 25 litre bottle hydrochloric acid 35 - 38argr grade in 25 litre bottle sodium hydroxide argr grade hydofluoric acid 40 argr grade in 25 litre bottle sodium thiosulphate ar grade.
Preparation of Organic Compounds. Sodium thiosulphate and hydrochloric acid Here is a suggested method to investigate the effect of varying the concentration of sodium thiosulphate. Acid solutions of sulphuric and hydrochloric acids containing ferrous salts from steel pickling.
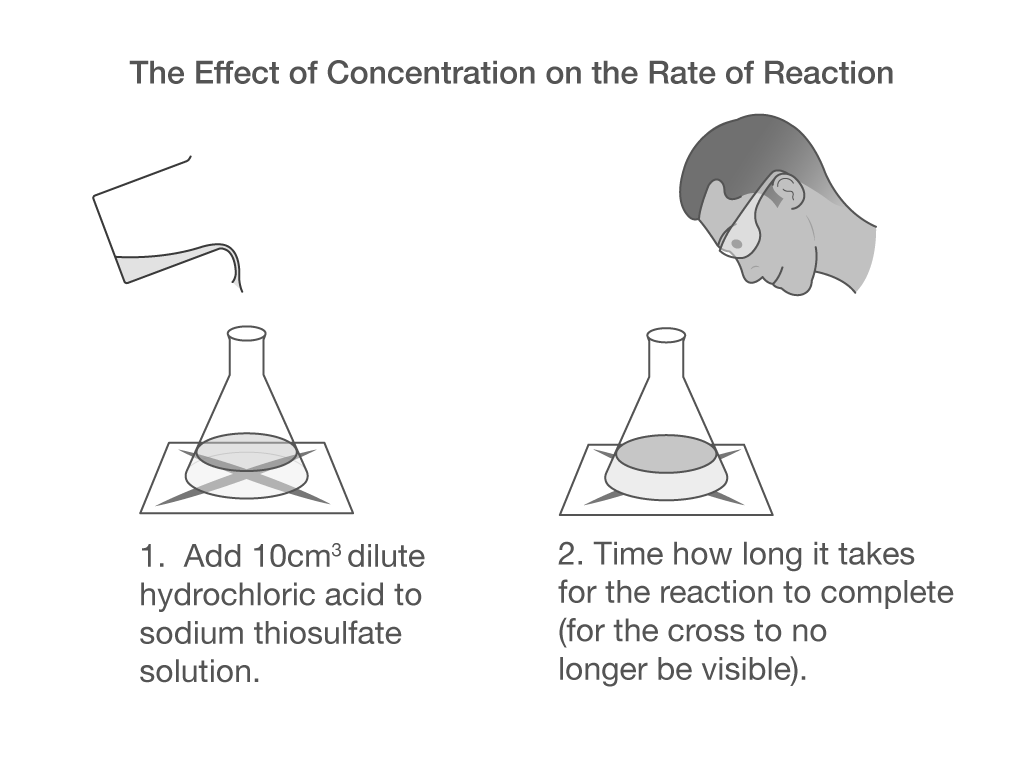
If you add dilute hydrochloric acid to sodium thiosulphate solution you get the slow formation of a pale yellow precipitate of sulphur. The sulphur forms in very small particles and causes the solution to cloud over and turn a yellow colour. Effectiveness of Different Common Oils in Forming Emulsions.
Hydrochloric acid 6 M potassium iodate potassium iodide sodium carbonate sodium thiosulfate pentahydrate soluble potato starch sulfuric acid 3 M Chlorox or similar liquid bleach as an unknown Preparation of Standard Sodium Thiosulfate Solution 1. Sodium tripolyphosphate Tripolyphosphate de sodium Pentasodium triphosphate Sodium triphosphate. The reaction of sodium thiosulphate and hydrochloric acid can be easy to study the effect of concentration on the rate of reaction.
Ethylenediamine tetra-acetic acid edta Ethyl Acetate. Hydrochloric acid 10 Hydrochloric acid 30 Hydrochloric acid 35 Hydrocyanic acid Hydrocyanic acid saturated Hydrofluoric acid 40 Hydrofluoric acid 60 Hydrofluoric acid 75 S S S S S S S S S S S S S S S S Methylene chloride 100 Methylsulfuric acid Milk Mineral oils Molasses Mustard prepared N U S S S S S U S S U S S Hydrogen. It is a by-product of chlorine manufacture along with sodium hypochlorite and sodium hydroxide.
Sulphuric acid 005 mol l-1 Dilute 31 ml sulphuric acid density 184 to 1 litre. The effect of temperature on this reaction can be measured by warming the sodium thiosulphate solution before adding the acid. The corrosion data in this section is mainly based on the results of general corrosion laboratory tests which are not strictly comparable with actual service conditionsThe corrosion tables provide an initial guide to the selection of materials and are intended to facilitate understanding of the different types of corrosion damage that can arise due to poor material selection.
Hydrochloric acid HCL is a clear colourless and pungent solution created by dissolving hydrogen chloride gas in water. For more information click on the link that follows. When Sodium Thiosulphate reacts with hydrochloric acid sulphur is produced.
Hydrochloric acid is colorless sodium thiosulphate is a white crystal The two will react to form a sodium chloride salt sulfur water and sulfur dioxide gas sulphur is a solid with an intense yellow color. There is a very simple but very effective way of measuring the time taken for a small fixed amount of precipitate to form. Education Resource Assistant Note - you must apply for this post via your local jobcentre using the following reference.
S S Sodium thiosulphate S S Potassium cyanide saturated S S Soybean oil S S Potassium dichromate 40 S S Stannic chloride saturated S S Potassium. This causes the cross to fade and eventually disappear. Add more about 700 ml of water mix.
How many conical flasks are required for this experiment. Add about 25 gm of Sodium Thiosulphate with continues stirring. Sodium thiosulphate normal solutions.
L Glutamic Acid hcl. Spermacetti wax synthetic pure. Hydrochloric acid Acide chlorhydrique Muriatic acid.
Sodium Thiosulphate Solution Preparation. Sodium Thiosulphate And Hydrochloric Acid 2. The presence of finely divided calcium carbonate suspensions in some natural waters may.
Dissolve approximately 01 g of sodium carbonate in one liter of distilled water. Sorbalene cream 10 glycerine sorbic acid fg. You can measure the rate of the reaction by altering the concentration of sodium thiosulphate and measuring the time it takes for the solution to turn fully opaque.
112 - Acid solutions sludges and residues containing heavy metals Solutions of sulphuric hydrochloric and nitric acids containing copper nickel chromium zinc cadmium tin lead or other heavy metals. Hydrochloric acid is found naturally in gastric acid. The concentration of sodium thiosulphate solution in Experiment I is higher than that in Experiment II.
As the sodium thiosulphate solution is run in from a burette the colour of the iodine fades. Ethylenediamine tetra-acetic acid di-potassium salt. Sodium thiosulphate crystals tech.
Add about 02 gm of Sodium Carbonate with continues stirring. Hydrochloric acid 10 S S Methylene chloride 100 U U Hydrochloric acid 30 S S Methylsulfuric acid S S. Qualitative Analysis of Carbohydrates.
Take about 100 ml of water in a cleaned and dried 1000 ml volumetric flask. Amount usually one drop of 01 mol l-1 sodium thiosulphate solution. We have a Kickstart employment and training opportunity within our Early Years and Primary Education team as an Education Resource Assistant.
Ethylenediamine tetra-acetic Acid di-Sodium Salt. Stannous fluoroborate tech. You add the last few drops of the sodium thiosulphate solution slowly until the blue.
When hydrochloric acid and sodium thiosulphate react the solution turns cloudy. Hydrochloric sulphuric and nitric acids and sodium hydroxide are in common use in the. Tests for the functional groups.
2 g of granulated zinc is added to a mixture of 50 cm 3 of 02 mol dm-3 hydrochloric acid and 2 cm 3 of 1 mol dm-3 copperII sulphate solution at room conditions.
Theallpapers Com
Talkingaboutscience Com
Turton Uk Com
What Makes The Solution Of Sodium Thiosulfate And Hydrochloric Acid Smelly Quora

Hksciblog Blog Archive Dse Chem Follow The Rate Of Reaction Between Thiosulphate Ions And Hydrochloric Acid
Learning By Questions

Rate Of Reaction Temperature

The Rate Of Reaction Between Sodium Thiosulfate And Hydrochloric Acid Free Essay Example

