Litmus paper is used to test the pH balance of a liquid or substance. The chemical reaction involved in acid-base titration is known as neutralisation reaction.

Difference Between Alkali And Base Compare The Difference Between Similar Terms

Difference Between Endpoint And Equivalence Point About Science

Properties Of Base Difference Between Alkali And Base Acids Bases And Salts Part 04 Class 10 Youtube
An alkali is a base that dissolves in water.

Difference between alkali and base. 124 The BE is commonly derived from nomograms. Alkali-silica reaction ASR is of more concern because aggregates containing reactive silica materials are more common. Base excess BE refers to the difference between the observed and the normal buffer base concentration or expressed differently the amount of acid or base required to return the pH to 74 in the setting of a normal Paco 2.
One of two subsets is commonly chosen. As both Alkali metals and alkali earth metals are the first two groups in the periodic table the difference between alkali metals and alkaline earth metals is a subject of interest for any. A base on the other hand which is soluble in nature is termed as an alkali.
A soluble base is also called an alkali. In ASR aggregates containing certain forms of silica will react with alkali hydroxide in concrete to form a gel that swells as it adsorbs water from. If the meniscus sits between two lines it should end 005.
It involves the combination of H 3 O ions with OH-ions to form water. However water is the only inorganic substance that can exist in all three physical states naturally as liquid water ice or water vapor. Alkalis are usually defined as a subset of the bases.
The difference between alkali and base is mainly based on its solubility in water. An acid is a chemical species that can be neutralized by a base. The terms base and alkali are often used interchangeably particularly outside the context of chemistry and chemical engineering.
A base that dissolves in water is also known as an alkali. It is also important that we also go over molarity. Often they have a hard calcareous layer at 05 to 1 metre depth.
Most substances are either alkali or acid. The terms HOMO and LUMO are under the subtopic molecular orbital theory in general chemistry. This is a greatly oversimplified explanation of acid-base chemistry.
Example 1 Distillation of Methylene Di Chloride with water Explanation In a process the separation of Methylene Di Chloride with water using a liquid-liquid separation technique can be called as a unit operation Explanation In the above process only separation is taking place which will be done base on density difference. Molarity is the measure of concentration and is usually used in acids alkalis and other solutions. Difference between alkali and base.
It would depend on how they make it in their labs. The key difference between Homo and Lumo is that the HOMO donates electrons whereas the LUMO receives electrons. The alkali metals are solids at room temperature except for hydrogen.
The term HOMO stands for highest occupied molecular orbital while the term LUMO stands for lowest unoccupied molecular orbital. Honestly it isnt the cocoa that I would worry about. In acid-base titrations solutions of alkali are titrated against standard acid solutions.
Smallest difference in readings that can be measured. Though most varieties of grits can be theoretically interchanged with cornmeal masa or polenta you usually get better results when you use the specific type of grain called for in a recipe. A base is an aqueous substance that donates electrons accept protons or release hydroxide OH- ions.
There are various more specific definitions for the concept of an alkali. The key difference between alkali metals and alkaline earth metals is that all alkali metals have an electron in their outermost shell whereas all the alkaline earth metals have two outer electrons. Key Differences Between Acid and Base.
Molarity is measured in concentration- the amount of solute in a solution which is measured in moles. The estimation of an alkali solution using a standard acid solution is called acidimetry. What is an Acid.
Liquids that are volatile acids. Difference Between Acid and Base - Acid is a kind of chemical compound that when dissolved in water gives a solution with H ion activity more than purified water. However read the package carefully to distinguish between grits and cornmeal which is fine-textured processed corn used like flour and masa harina which is the base for tortillas.
What is the difference between Acid and Alkaline Comparison of Key Differences. Processed with alkali doesnt necessarily mean chemical processing but it can. Alkali or Alkaline soils are clay soils with high pH greater than 85 a poor soil structure and a low infiltration capacity.
Acidic substances include vinegar lemon juice and battery acid. Basic substances react to aqueous solutions by accepting protons giving away electrons or releasing hydroxide ionsThey neutralize acids by reacting with hydrogen ions to form salts and water. The differences listed above depicts the clear difference between acids and bases which forms part of the chemistry and discussed among students the.
Following are the important points which differentiate the acids to that of base. If acid is slowly added the solution remains yellow until all the alkali has. Difference between Acid and Base.
Volatile liquids acids when mixed with specific substances turn into salts. If the bottom of the meniscus sits between the. Alkaline or basic chemicals include baking soda ammonia and lye.
A second is defined as 9192631770 cycles of the radiation corresponding to the energy difference between the ground state and one of the excited states of the cesium-133 atom. Acid Alkali Alkali Earth Metals Alkaline Aquatic Chemistry Bases Lewis Acid pH Strong Acids Weak Acids. A base is a substance that can neutralize the acid by reacting with hydrogen ions.
Most Girl Scout cookies are heavily processed as is most boxed products like this. Chemical indicator any substance that gives a visible sign usually by a colour change of the presence or absence of a threshold concentration of a chemical species such as an acid or an alkali in a solutionAn example is the substance called methyl yellow which imparts a yellow colour to an alkaline solution. What is a base in chemistry.
Stainless steel fabrication and application aspects. These substances form a. On the other hand Bronsted-Lowry.
This is due. A reaction between an acid and a base is called neutralization and this neutralization results in production of water and a salt. 201 stainless steel has certain acid resistance alkali resistance performance high density polishing without bubbles no pinhole and other characteristics is the production of a variety of watchcases watchband base cover quality materials.
Furthermore these alkali metal amidoborane aided reactions is quite different from the reactions of ester with NH 3 NH 2 CH 3 or NaNH 2 as previously reported 14454647484950. Example of Unit operation. Alkali soils owe their unfavorable physico-chemical properties mainly to the dominating presence of sodium carbonate which causes the soil to swell and difficult to clarifysettle.
Acid is the substance when dissolved in water increases the concentration of H ions whereas the base is the substance when dissolved in water increase the concentration of OH ions. If you understand them lets move on. They are creating a more oreo like cookie when they process it with alkali.
If you forgot the difference click here. If the bottom of the meniscus sits on a line it should end with a 000 as in the above example 900cm3. According to Arrhenius concept.
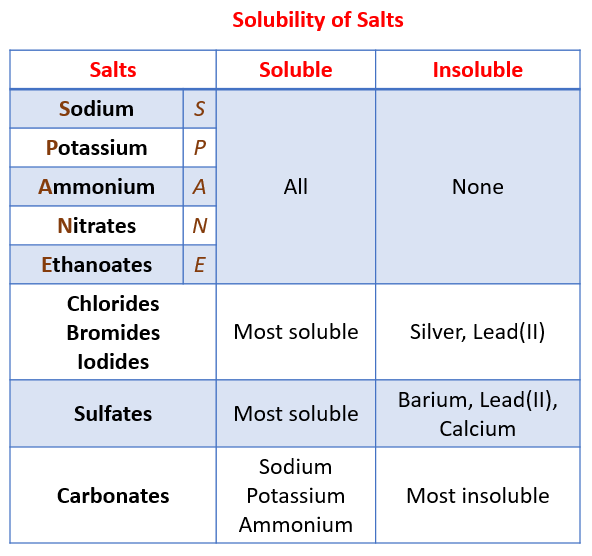
Acid Bases Salts Igcse Chemistry Solutions Examples Worksheets Videos
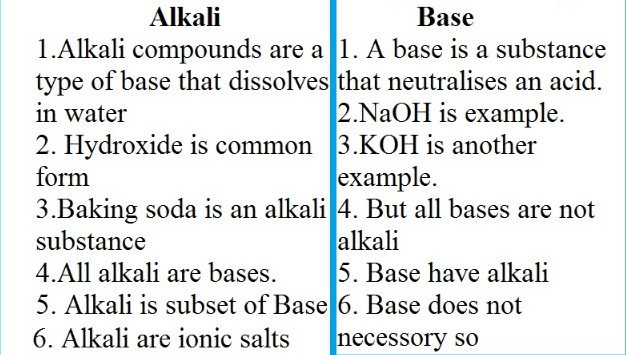
Expalin The Basic Differences Between Base And Alkali With Table

Chemical Properties Of Alkalis O Level Secondary Chemistry Tuition

Question Video Identifying The Difference Between Alkalis And Bases Nagwa

Difference Between Base And Alkali Insight Youtube

Difference Between Alkali And Base With Table Ask Any Difference

Difference Between Alkali Metals And Alkaline Earth Metals Compare The Difference Between Similar Terms
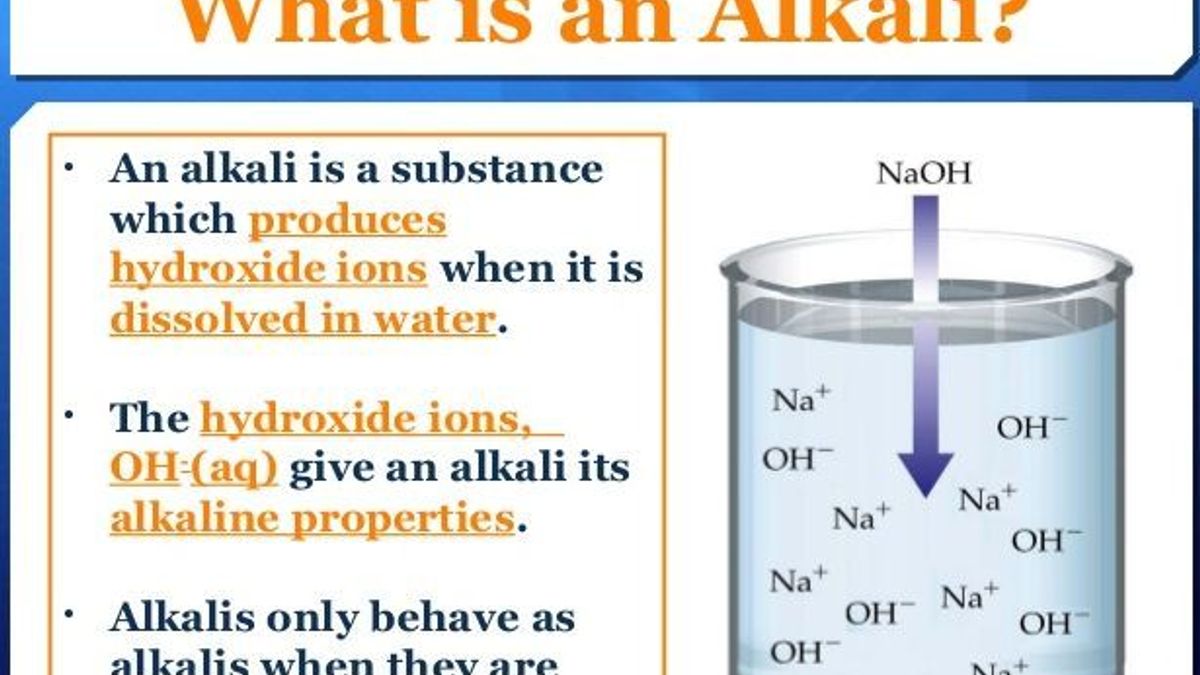
What Is An Alkali And Alkaline Solution

